Properties of an Atom
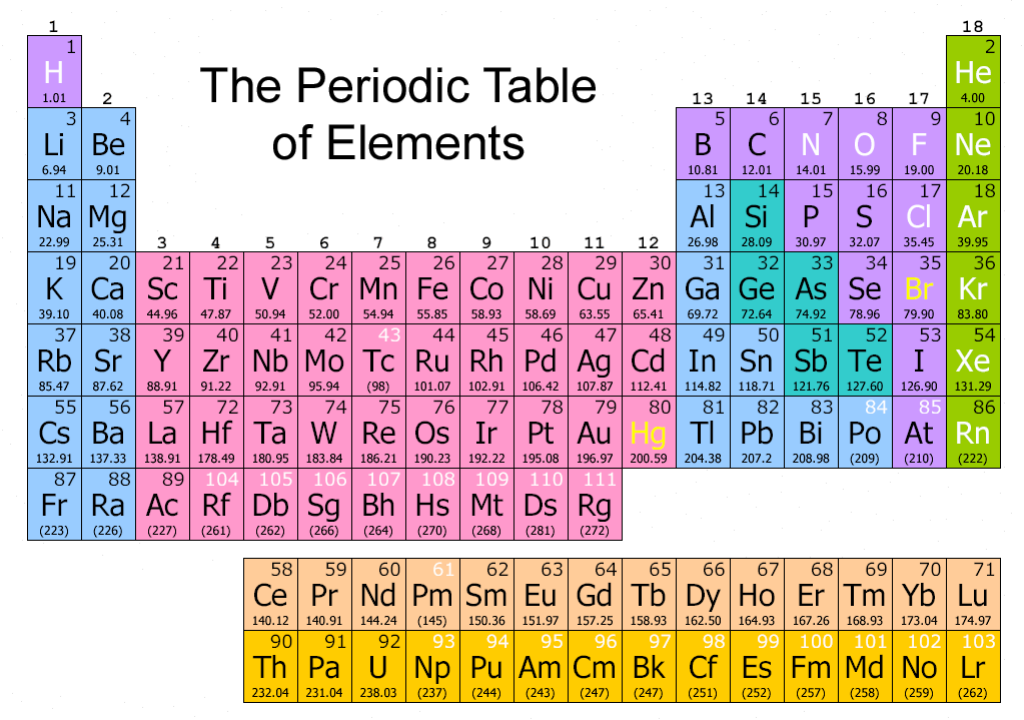
Atomic Number
The atomic number tells you how many protons an atom has. For example, every hydrogen atom has the atomic number 1 because it only has 1 proton.
Elements that have atomic numbers of up to 92 can be found in nature; those over 92 are created by scientists in a laboratory.
The atomic number tells us where we can find an element in the periodic table. This table shows all the atoms in groups.
Atomic Mass
The atomic mass is the number of protons and neutrons in an atom. Although all atoms of the same element have the same number of protons, they sometimes have more neutrons. Such atoms are called isotopes.
For example, hydrogen has three isotopes. Most of the time a hydrogen atom has one proton and one neutron. Sometimes you can find hydrogen isotopes that have two or three neutrons, but they too have only one proton.
In most lighter elements, the nucleus of each atom has the same number of protons and neutrons, but heavier elements have more neutrons than protons. Uranium , for example, has 92 protons and 146 neutrons. It’s atomic mass is 238.
The atomic mass is never a whole number, because scientists do not just add protons and neutrons together. They use a complicated formula.
Electric charge
Normally, an atom is electrically neutral. But it can gain or lose electrons when it crashes with other atoms. Atoms that gain or lose electrons are called ions. They have an electric charge.
Atoms that lose electrons become positive ions; atoms that win electrons become negative ions.
Radioactivity
In some atoms, the nucleus can change naturally. Such an atom is radioactive. When a nucleus changes, it produces rays.
In nature, there are some elements that are radioactive, like uranium or radium. In labs scientists can produce radioactivity by bombarding atoms with smaller particles.